AnswerExplanation:
The liters of a 6.85 M solution that should be used to make the solution can be calculated using the dilution formula.
The formula for calculating dilutions is:
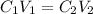
C₁ = 2.45 M
V₁ = 7837 mL = 7837/1000 = 7.837 L
C₂ = 6.85 M
V₂ = unknown
The unknown volume, V₂ is the required volume needed to make the solution.
Therefore, the V₂ can be calculated using the formula above as follows:
![undefined]()