Answer:
Option C and D are correct.
Explanations:
What is electron affinity?
Electron affinity is dfined as the energy required to remove en electron from a gaseous atom or molecule. An example of a gaseous atom loosing an electron is given as:
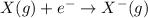
From the reaction, you can see that the gaseous element X looses an electron from its outershell to form an ion. This reaction is known as the first electron affinity.
Hence the correct statement dealing with electron affinity are:
• It is the enthalpy change associated with the, removal of an electron, from a gaseous atom or ion.
• In general the, first electron affinity, is:, X(g) + e- → X-(g)