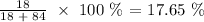Answer:
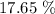
Step-by-step explanation:
Here, we want to get the percentage by mass of the water molecules in the given compound
To get this, we have to divide the molar mass of water by the molar mass of the percentage and write the answer as a percentage
The molar mass of water is 18 g/mol
The molar mass of MgCO3 is 84 g/mol
Thus, the percentage by mass of water will be: