Answer:
197.12L of N2 are required.
Step-by-step explanation:
1st) From the balanced reaction we know that 1 mol of N2 reacts with 3 moles of H2 to produce 2 moles of NH3.
2nd) It is necessary to convert moles to grams with the molar mass, and use the stoichiometry of the balanced reaction to find the amount of N2 moles that are required to produce 300g of NH3:
- N2 molar mass: 28g/mol
- NH3 molar mass: 17.01g/mol
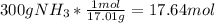
300g of NH3 is equal to 17.64 moles of NH3.
3rd) Now, with a mathematical rule of three we can find the moles of N2 that are required to produce 17.64 moles of NH3:
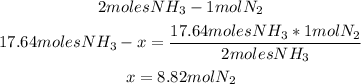
Now we know that 8.82 moles of N2 are needed.
4th) Finally, using the molar volume value (22.4L), we can calculate the liters of N2 that are required.
The molar volume indicates that 1 mole is equivalent to a volume of 22.4L under normal conditions.
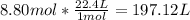
So, 197.12L of N2 are required.