Answer:
184g
Explanations:
The formula for calculating the mass of a substance is expressed as;
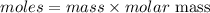
Given the following parameters
• Moles of Na = 8moles
,
• Molar mass of Na = 23g/mol
Substitute the given parameter into the formula
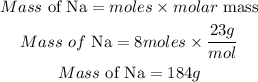
Hence the mass of the sodium is 184g