Answer:
The factor of change is 1/4.
Step-by-step explanation:
Since in this case we have a constant temperature, the changes in the pressure will affect the volume like the Boyle's Law formula explains:
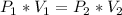
To find the change of the sample volume, let's replace the initial pressure (P1) with A, and let's increase the pressure (P2) 4x:
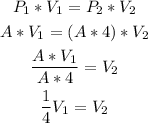
So, the factor of change is 1/4.