Answer:
3N of sulfuric acid.
Step-by-step explanation:
Remember that normality is a measure of concentration equal to the gram equivalent weight per liter of solution.
First, let's see the equation of normality:
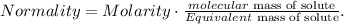
And let's see that sulfuric acid (H2SO4) is a diprotic acid because it contains 2 protons in its structure (2H+), so we're going to use this value to calculate the equivalent mass of solute.
Now, let's replace the given data: molarity is 1.5 M, the molecular mass of solute is the molecular mass of H2SO4 which is 98 g/mol (you can calculate the molecular mass of a compound using the periodic table) and the equivalent mass of solute is the division between molecular mass and the number of protons, in this case, 2:
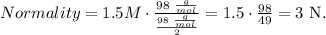
The normality of this solution would be 3N.