1) List the known and unknown quantities.
Concentration: 16%(m/m).
Mass of solute: 10 g NaOH.
Mass of solvent: unknown.
2) Set the equation.
Mass percent.
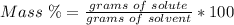
3) Plug in the known values and solve for grams of solvent (water).
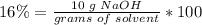
.
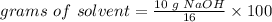
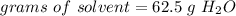
The 16%(m/m) solution contains 62.5 g H2O.
.