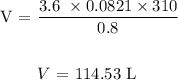Answer:
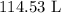
Step-by-step explanation:
Here, we want to get the volume of the gas inside the balloon
Mathematically, from the ideal gas equation:
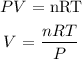
where:
P is the pressure which is given as 0.8 atm
V is the volume which we want to calculate
R is the molar gas constant which is 0.0821 L.atm/mol.K
T is the temperature in Celsius which we can convert to K by adding 273 (273 + 37 = 310 K)
n is the number of moles which is 3.6
Substituting the values, we have it that: