Answer
6.312 M
Step-by-step explanation
Given:
Volume of Mg(OH)₂, Vb = 53.96 mL
Volume of HCl, Va = 93.24 mL
Molarity of HCl, Ma =7.306 M
What to find:
The molarity of Mg(OH)₂, Mb
Solution:
The first step is to write a balanced chemical equation for the reaction.
Mg(OH)₂ + 2HCl → MgCl₂ + 2H₂O
Mole ratio is 1:2; that is na = 2 and nb = 1
Hence, the molarity of Mg(OH)₂, Mb is calculated using the formula below:
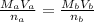
Plugging the values of the given parameters into the formula, we have:
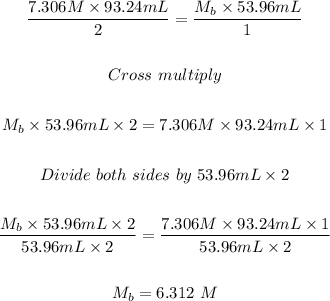
Therefore, the molarity of a 53.96 mL magnesium hydroxide solution that is required to neutralize 93.24mL of a 7.306 M solution of the hydrochloric acid solution is 6.312 M