Answer:
-163kJ/mol
Explanations:
Given the reaction between the zinc metal reacts with HCl according to the balanced equation:
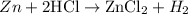
The required heat of reaction will be calculated using the formula below:
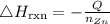
where:
• Q is the ,heat produced
,
• nZn is the, number of moles that reacted
The formula for calculating the quantity of heat produced is expressed as:
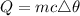
where:
• m is the ,mass, of the ,Zinc metal
,
• c is the ,specific heat capacity, of zinc
,
• △θ is th,e change in temperature
Get the mass of zinc
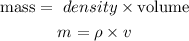
The quantity of heat becomes:
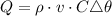
Substitute the given parameters to have:
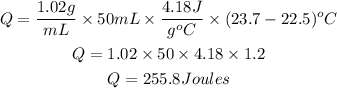
Next is to get the number of moles of Zinc that reacted (nZn)
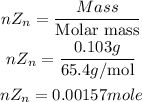
Get the required heat of reaction of Zinc:
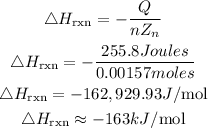
Hence the heat of the reaction of Zinc is approximately -163kJ/mol