Answer:
30.4 %.
Step-by-step explanation:
Let's see the volume percentage (% v/v) formula:
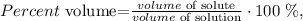
in this case, the solute is acetic acid and the solution is the solute and solvent (which is water). Based on this logic, the volume of solute is 17.8 mL, whereas the volume of solution is the sum of the volumes of solute and solvent ( 17.8 mL + 40.8 mL = 58.6 mL), so we just have to replace the values that we have in the formula:
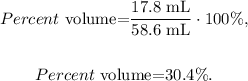
The answer would be 30.4 %.