ANSWER
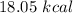
Step-by-step explanation
The amount of heat necessary to change the ice to water is given by:
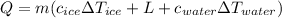
where m = mass of ice = 480 g = 0.48 kg
c(ice) = specific heat capacity of ice = 2108 J/kg/K
ΔTice = change in temperature of ice = 0 - (-19) = 19 K or 19 °C
L = latent heat of fusion of ice = 33600 J/k
c(water) = specific heat capacity of water = 4186 J/kg/K
ΔTwater = change in temperature of water = 20 - 0 = 20 K or 20 °C
Therefore, the heat necessary is:
![\begin{gathered} Q=0.48([2108*19]+33600+[4186*20]) \\ \\ Q=0.48(40052+33600+83720)=0.48*157372 \\ \\ Q=75538.56\text{ }J \end{gathered}](https://img.qammunity.org/2023/formulas/physics/college/7pb23nbd5q8q7hf4nuwjsdpi22o8x7wyww.png)
Convert this to kcal:
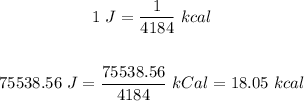
That is the answer.