Its volume will be 12-fold.
From the Ideal Gas formula we know that:
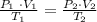
- P is the pressure os the gas.
- V is the volume of the gas.
- T is the temperature of the gas.
So, we can replace the hypothetical values to calculate the volume of the gas:
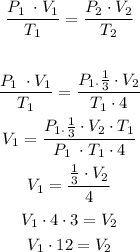
Finally, its volume will be 12-fold.