To calculate the calories that the water gained, we must use the specific heat of the water. The specific heat of water is 1cal/g°C, which means that it takes one calorie to raise one gram of water 1°C.
Now, we need the mass of water and the temperature difference.
We will calculate the mass of water from its density, the density of water is 1mL/g. Density is defined as:
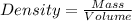
We clear the mass and replace the known data:
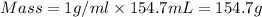
Now, the temperature difference will be:
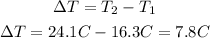
Calories gained by water are calculated from the following equation:
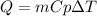
where,
Q is the heat or energy that the substance absorbs or releases. In this case, the water will absorb energy.
m is the mass of water = 154.7g
Cp is the specific heat of water, 1cal/g°C
dT is the difference of temperature, 7.8°C
Now, we replace the known data:
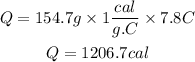
Answer: The water gained 1206.7 calories of heat