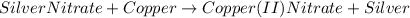The reaction of silver nitrate with copper is a substitution reaction. We have copper in its free state, Cu, and we have silver nitrate which has the formula AgNO3. The copper replaces the silver obtaining the following reaction:
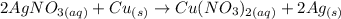
The products obtained are copper nitrate and silver.
The word equation will be: