Using the fact that:
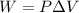
We could replace our values as follows:
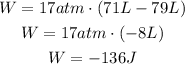
As you can see, the work done is negative since the gas is being compressed. Therefore,
The work done is -136J.
In kJ, the answer is -0.136kJ. Rounded to 2 significant digits, this is -0.14kJ