Answer
250 mL of 0.125 M HCl can be prepared from concentrated HCl (aq) that is 38.0% by mass with a density of 1.19 g/mL by adding 2.49 mL of HCL(38%) into a small quantity of water, mix to disperse; then dilute with solvent water up to but not to exceed the total needed volume of solution (in this case 250 mL).
Step-by-step explanation
The problem can be solve in four steps:
Step 1: Calculate the number of moles in 38.0% by mass of HCl.

Step 2: The volume of the 38.0% by mass HCl solution.
Volume of solution in Liters containing 1.06 mole of HCl =
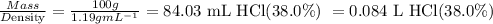
Step 3: Calculate the molarity of HCl (38.0%)
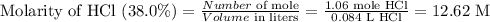
Step 4: To calculate the volume of HCl (38.0%) required to prepare 250 mL of 0.125 M HCl.
Note: Formula weight of HCl = 36 g/mol

Therefore, 250 mL of 0.125 M HCl can be prepared from concentrated HCl (aq) that is 38.0% by mass with a density of 1.19 g/mL by adding 2.49 mL of HCL(38%) into a small quantity of water, mix to disperse; then dilute with solvent water up to but not to exceed the total needed volume of solution (in this case 250 mL).