Given:
The specific heat of the material is c Cal/(gram°C)
The mass of the material, m=5 g
The initial temperature of the material, T₁=10 °C
The final temperature of the material, T₂=37 °C
To find:
The expression for the amount of heat added to the material.
Step-by-step explanation:
The specific heat of a material is the heat required to raise the temperature of the unit mass of the material by one degree celcius.
From the equation of specific heat, the amount of the heat added to a material is given by,
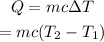
On substituting the known values,
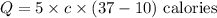
Final answer:
Thus the correct answer is option A.