To calculate the molecules present in the given moles we will apply for Avogadro's number. Avogadro's number tells us that in one mole of any substance there are 6.022x10^23 molecules.
Therefore if we have 4 moles of NaCl we will have:
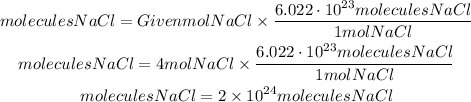
In 4 moles of NaCl there are 2 x10^24 molecules