Answer:
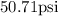
Explanations:
According to Gay Lussac's law, the pressure of a given mass of gas is directly proportional to its temperature provided that volume is constant. Mathematically;
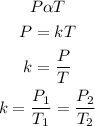
From the given question, we are given the following parameters:
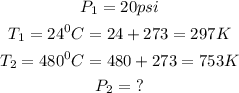
Substitute the given parameters into the formula as shown:
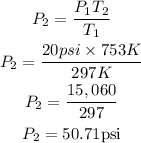
Therefore the pressure of the spray paint at 480 celsius is 50.71psi