ANSWER
Step-by-step explanation
Given that
The number o molecules is 6.02 x 10^23 molecules
Firstly, find the number of moles using the below formula
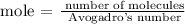
Recall, that the Avogadro's number is 6.02 x 10^23
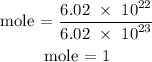
Secondly, find the mass of CO2 using the formula below
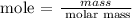
Recall, that the molar mass of CO2 is given as 44.01 g/mol
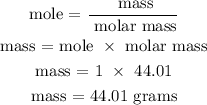
Therefore, the mass of CO2 is 44 grams