Answer:
267 mmHg.
Step-by-step explanation:
What is given?
Total pressure = 723 mmHg
Partial pressure of helium (He) = 194 mmHg.
Partial pressure of nitrogen (N) = 262 mmHg.
Step-by-step solution:
Let's see the formula of total pressure:
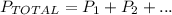
Let's write the formula to our context if we have 3 different gases:
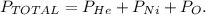
We want to find the partial pressure of oxygen (O), so let's solve for Po which is the unknown value in the formula, and replace the given data:
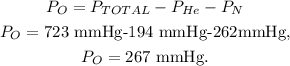
The partial pressure of oxygen is 267 mmHg.