A chemist is using 383 milliliters of a solution of acid and water.
If 17.3% of the solution is acid, how many milliliters of acid are there?
We basically need to calculate 17.3% of 383 milliliters.
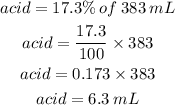
Therefore, the solution has 6.3 milliliters of acid.