Step 1
The reaction:
1 A (aq) + 1 B (s) <=> 2 C (aq) + 1 D (aq)
A, C and D participate in the equilibrium constant K
B doesn't participate
--------------------
Step 2
Data provided:
At equilibrium:
[A] =4.5 x 10^-4 M
[C] = 1.2 x 10^-2 M
[D] = Unknown
-------------------
Step 3
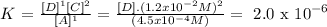
Answer:
[D] = 6.25x10^-6 M