the amount of potassium-42 remaining after 62 hours is approximately 23.31 grams (option B)
Step-by-step explanation:
half life = 12.4 hours
initial amount = 746g
time elapsed = 62 hours
Using the half-life formula:
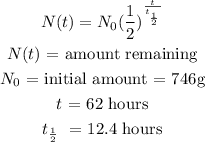
Substitute for the values:
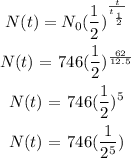
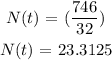
Hence, the amount of potassium-42 remaining after 62 hours is approximately 23.31 grams (option B)