Answer:
The reaction will occur.
![CuSO_(4)+Na_(2)CO_(3)\operatorname{\rightarrow}Na_(2)SO_(4)+CuCO_(3)]()
Explanations:
The given chemical reaction is a double displacement reaction. In this reaction, the compoiunds will react to produce two different compounds.
The balanced form of the reaction is expressed as:
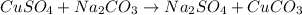
You can see that the reaction of cupper(II)sulphate and sodium carbonate produces sodium sulphate and copper(II)carbonate
Since most carbonate compounds are insoluble, hence the reaction will occur