Given:
The hydrogen ion concentration of a solution is 0.0001 mol/L
we will find the value of pH
The relation between pH and the hydrogen ion concentration H will be:
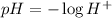
Given H = 0.0001 mol/L
so, the value of pH will be as follows:
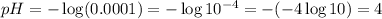
so, the answer will be pH = 4