Given:
The specific heat capacity of steel is
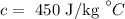
The mass of the steel is m = 2 kg
The initial temperature of steel is
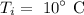
The final temperature of steel is
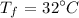
The time is t = 30 s
To find the heat flow rate.
Step-by-step explanation:
The heat flow rate can be calculated by the formula
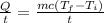
On substituting the value, the heat flow rate will be
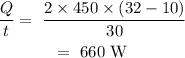
Thus, the heat flow rate is 660 W.