1) List the known and unknown quantities.
Molar mass: 34.04 g.
Empirical formula: NH.
Molecular formula: unknown.
2) Find the molar mass of the empirical formula.
N: 14.007 g/mol.
H: 1.008 g/mol.
N + H = 14.007 + 1.008 = 15.015 g/mol.
3) Divide the molar mass by the molar mass of the empirical formula
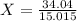
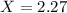
Multiply the subscripts of the empirical formula by 2.
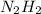
.