Given:
The half-life of carbon-14 is 5730 years.
The initial amount of carbon is I = 50 grams.
Step-by-step explanation:
To find the final amount of carbon after 1000 years.
The fundamental decay equation is,
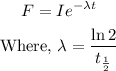
Let us find the radioactive constant first.
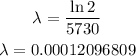
Then, the final amount of the corban-14 is,
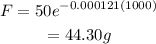
Hence, the amount of a 50-gram sample of Carbon-14 will be left in 1000 years is 44.30 g.