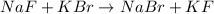Step-by-step explanation
Let us first define the different types of reaction.
A combination reaction is one in which two reactants combine to form a single product.
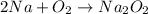
A decomposition reaction will be one in which a reactant breaks down into two or more products.
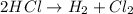
A simple displacement reaction will be one in which a reagent, which is in its natural state, displaces one element bound to another. As a product, we will have the previously linked element now in its free form.
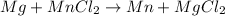
In a double displacement reaction two linked elements move past each other and take each other's place.