We first verify that the reaction equation is balanced. To do this we have the atoms of each element on each side of the reaction. we have 2 potassium atoms, 2 chlorine atoms, and 6 oxygen atoms on each side of the reaction. So the equation is balanced.
Now, by stoichiometry we can find the moles of KCl. The KCl to O2 ratio is 2/3 so the moles of KCl will be:
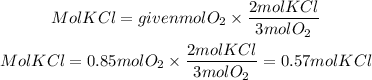
The moles of potassium chloride produced is 0.57mol KCl