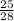Step 1. There are 5 red marbles and 3 blue marbles, 8 marbles in total:
Step 2. Two marbles are drawn simultaneously, which means that there is no replacing of the marbles.
To make the tree diagram with the probabilities for each marble, remember the probability formula:
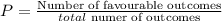
The three will look as follows:
For the first marble, the probability of getting a red one is 5/8 because there are 5 red marbles out of a total of 8, and similar to the first one is blue.
For the second marble, we consider that the total number of marbles is now 7.
Step 3. To find the probability of each combination, we multiply the values of each branch:
The results of the multiplications is:
The ones that have at least one red marble are the first three branches: red-red, red-blue, and blue-red.
To find the probability of at least one red marble, we add the results of the first three branches:
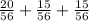
The result is:
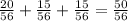
The probability is 50/56.
The fraction can be simplified to:
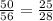
The probability simplified is 25/28.
Answer: