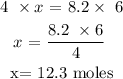Answer:
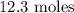
Step-by-step explanation:
Here, we want to get the number of moles of water that would form
From the equation of reaction, 4 moles of ammonia produced 6 moles of water
However, 8.2 moles of ammonia will produce x moles of water
To get the value of x, we have it that: