Answer: 8.1297x10^24 molecules
Calculations:
Balanced Reaction :
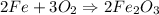
1. by stoichiometry,
• 2 moles of Fe : 2 moles of iron (iii) oxide
• then 13.5 moles Fe : x
X = 13.5 *2 /2 = 13.5 moles of Fe
• This means that 13.5 mole of Fe2O3 will be produce.
2. Calculate number of molecules by Avogardo principles.
No. of molecules = Moles * Avogardo Number
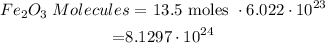
• This means that there are 8.1297 x10^24molecules of Fe2O3 from 13.5 moles Fe.