Answer
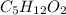
Step-by-step explanation
Given:
57.69% C, 11.54% H, and 30.77% O by mass,
Meaning:
Mass of carbon = 0.5769 g
Mass of Hydrogen = 0.1154 g
Mass of Oxygen = 0.3077 g
We know:
Molar masses of:-
C = 12.0107 g/mol
H = 1.00794 g/mol
O = 15.999 g/mol
Required: Empirical formula
Solution:
Step 1: Calculate the number of moles of the 3 atoms
C : 0.5769 g/12.0107 g/mol = 0.04826 mol
H : 0.1154 g/1.00794 g/mol = 0.1145 mol
O : 0.3077 g/15.999 g/mol = 0.0192
Step 2: Divide the moles by the lowest number of moles
In this case divide by 0.0192
C: 2.5
H: 6
O:1
Step 3: multiply by 2 to remove a decimal
C: 5
H: 12
O:2
Therefore the answer is: C5H12O2