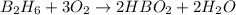
From the chemical equation we see that 1 mole of diborane (B2H6) will produce 2 moles of water.
We will firstly calculate the moles of diborane:
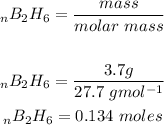
One mole of diborane produces 2 moles of water then to find the moles of water that is produced we:
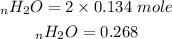
We then convert the moles of the water to mass:
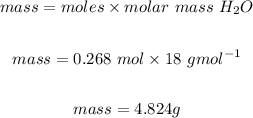
Mass of water is 4.824g