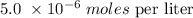Answer:
Step-by-step explanation:
By definition, the pH of a solution is the negative logarithm to base 10 of the concentration of Hydroxonium or oxonium ion
Mathematically, we have this as:
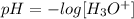
We can change the formula subject so we would have hydroxonium ion on the left
Mathematically, we have this as:
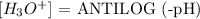
Finally, we have the calculation as follows:
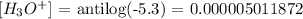
In the scientific form,we have this as: