The question requires us to calculate the energy of a photon given that its frequency is 7.87 × 10^15 Hz.
To calculate the energy of a photon, we can use the following equation:
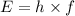
where h is the Planck's constant (6.626 x 10^-34 Jxs) and f is the frequency (in Hz or 1/s). (Note that the unit Hz is equivalent to s^-1)
Then, applying the frequency provided and the Planck's constant to the equation, we have:
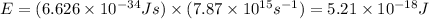
Therefore, the energy of the photon is 5.21 x 10^-18 J.