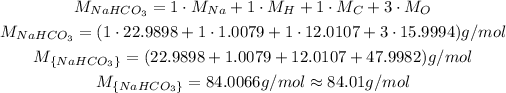To calculate the molar mass of NaHCO₃, we need to add the molar mass of each of its atoms.
This compound has 1 atom of Na, 1 of H 1 of C and 3 of O.
The molar mass of these atoms can be consulted on a periodic table, and they are:
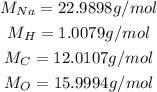
So, the molar mass of NaHCO₃ is: