Answer:
0.96moles
Explanations:
Given the reaction between Magnessium and silver nitrate expressed as:
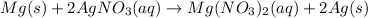
Given the following parameter
Moles of Magnessium at the start = 0.480moles
According to stoichiometry, 1mole of magnessium produce 2 moles of silver. Hence the moles of silver that is produced if 0.48moles of Mg reacted is given as:
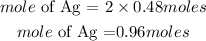
Hence the moles of silver that will produced is 0.96moles