Answer:
12.5 %.
Step-by-step explanation:
Let's see the half-life formula:
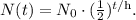
Where N₀ is the initial amount, t is time, and h is the half-life.
We want to know what would be the percentage of a sample of iodine-131 that remains. This is the same that N(t)/N₀, so we have to replace the given data in the formula and multiply it by 100 because it is a percentage.
The half-life of iodine-131 is 8.0 days, and 3 half-lives are equal to 24 days (8.0 x 3 = 24):
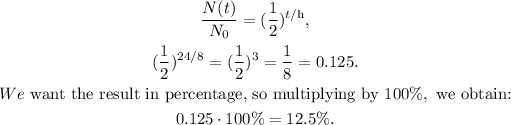
The answer would be 12.5 %.