Answer;
pH = 7
pOH = 7
Explanations:
The formula for calculating the pOH of a solution is given as:
![pOH=-log[OH^-]](https://img.qammunity.org/2023/formulas/chemistry/college/vqo02fhb0jj8u1geaydn77je0d54m8v5tf.png)
Given the following parameter
![[OH^-]=1.0*10^(-7)M](https://img.qammunity.org/2023/formulas/chemistry/college/5oogg1awthdx1vr4dfchbx6fnrq8iznvbe.png)
Substitute
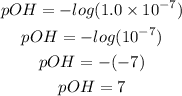
Determine the pH of the solution
Recall that pH + pOH = 14, hence;
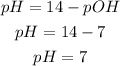
Hence the required pH and pOH of aqueous solution are 7 and 7 respectively