ANSWER
The volume of the acid is 0.5 L
Step-by-step explanation
Given that;
The molarity of HCl is 1.00 M
The volume of Na2CO3 is 0.500 L
The molarity of Na2CO3 is 0.500M
To find the volume of HCl needed, follow the steps below
Step 1; Write the balanced equation for the reaction
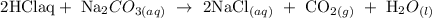
In the above reaction, 2 moles of HCl is needed to completely react with Na2CO3 .
Step 2; Apply the molarity concept to find the volume of the acid
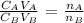
Where
CA is the concentration of the acid
VA is the volume of acid
CB is the concentration of the base
VB is the volume of the base
nA is the number of moles of acid
nB is the number of moles of base
Step 3; Substitute the given data into the formula in step 2
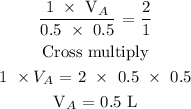
Hence, the volume of the acid is 0.5 L