Part A)
According to the text, the equation that relates x and y is:
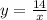
Substitute x=21 to find the value of y when x=21:
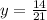
Simplify the expression:
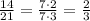
Therefore, the value of y when x=21 is:
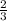
Part B)
To find the value of x when y=28, substitute y=28 and solve for x:
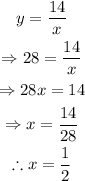
Therefore, the value of x when y=28 is:
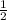
Part C)
Since the pressure P is inversely proportional to the volume V, then:
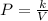
Solve for k and substitute P=250kPa and V=1.7m^2 to find the constant of proportionality:
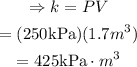
Therefore, the constant of proportionality for this situation is:
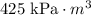
Part D)
Substitute the value of k into the equation that shows the inverse relation between P and V:
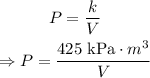
Therefore, the inverse variation equation model for this situation, is:
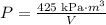
Part E)
Substitute V=3.2m^3 to find the pressure under those conditions:

Therefore, the pressure would be:
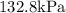
Part F)
Isolate V from the equation and substitute P=150kPa:

Therefore, the approximate volume would have to be equal to:
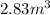
Part G)
Since the temperature has decreased, the pressure must be lower according to the description provided in the text. Then, an inequality to model this situation would be:
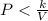
Part H)
Substitute the value of k and V=3m^3:
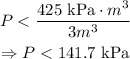
Part I)
Mathematically, all numbers under 141.7 satisfy the inequality from part H. Nevertheless, negative pressures do not have a physical meaning under the context of the Ideal Gas Law. Therefore, we must include the condition that P is greater than 0:
[tex]0