Step 1 - Understanding the definition of pOH
pOH is defined as:
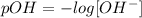
I.e., we can calculate the pOH by knowing the concentration of OH- in the solution.
Step 2 - Calculating the pOH of the given solutions
a) The first solution has a OH- concentration of 4.3 x 10-6 M. We just need to set this value in the definition of pOH:
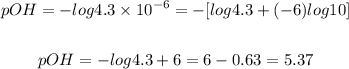
b) The second solution has a OH- concentration of 2.0 x 10-6 M. Let's calculate its pOH:
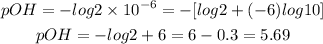
c) The third solution has a OH- concentration of 4.5 x 10-11 M. Let's calculate its pOH:
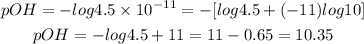
Answer: the pOH of the solutions are, respectively, 5.37, 5.69 and 10.35