We are given the following information
Mass of water = 25.5 g
Initial temperature of water = 29.3 °C
Final temperature of water = 43.87 °C
The specific heat capacity of water is 4.186 J/g.°C
The amount of heat required is given by
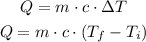
Let us substitute the given values into the above formula
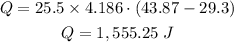
Therefore, we need 1,555.25 Joules of heat to bring 25.5 g of water from 29.3 °C to 43.87 °C.