Given,
Initial volume of the water, V₁=3.00 m³
The initial temperature of the water, T₁=20.0 °C=293.15 K
The final temperature of the water, T₂=60 °C=333.15 K
From Charle's law, we have,
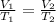
On rearranging the above equation,
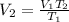
On substituting the known values in the above equation,
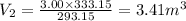
Therefore the change in the volume is,
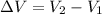
i.e.,
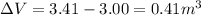
Therefore, the volume of the water will expand by 0.41 m³