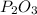The empirical formulas refer to the formulas where the ratios are the simplest, while the molecular formula shows the number of each type of atom.
In this case, we have a molecular formula and we just have to simplify the number to get the empirical formula. Divide each number by 2.
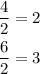
Therefore, the empirical formula is